Explore New Macular Degeneration Treatment Options
uinting, moving the page back and forth, and quietly panicking. Later, they were diagnosed with age-related macular degeneration (AMD).
Fast-forward a few years, and I’ve now sat in retina clinics, talked with ophthalmologists, watched injections being prepared, and tested some of the new tools and supplements myself. Macular degeneration went from a vague “eye disease” in my vocabulary to something I can explain in nerdy detail at family dinners. (My family is very patient.)
If you or someone you love has heard the words “dry” or “wet” AMD and walked out of the office feeling overwhelmed, you’re not alone. Let’s unpack what’s actually new—and what’s genuinely promising—without pretending there’s a magic cure.
Quick refresher: what macular degeneration actually is
AMD is damage to the macula, the tiny central part of the retina that gives you sharp, detailed vision. When it’s damaged, you may have:
- Blurred central vision
- Dark or empty spots in the center of your sight
- Distorted lines (that “wavy lines” complaint)
There are two main forms:
- Dry AMD (most common): Gradual damage, drusen deposits, thinning of the macula (geographic atrophy in advanced stages).
- Wet AMD (less common but more severe): Abnormal blood vessels grow under the retina, leak fluid or blood, and can rapidly destroy central vision.
When I started digging into research, one thing stood out: we’re not talking about a rare disease. The CDC estimates about 19.8 million adults in the U.S. have some form of AMD.
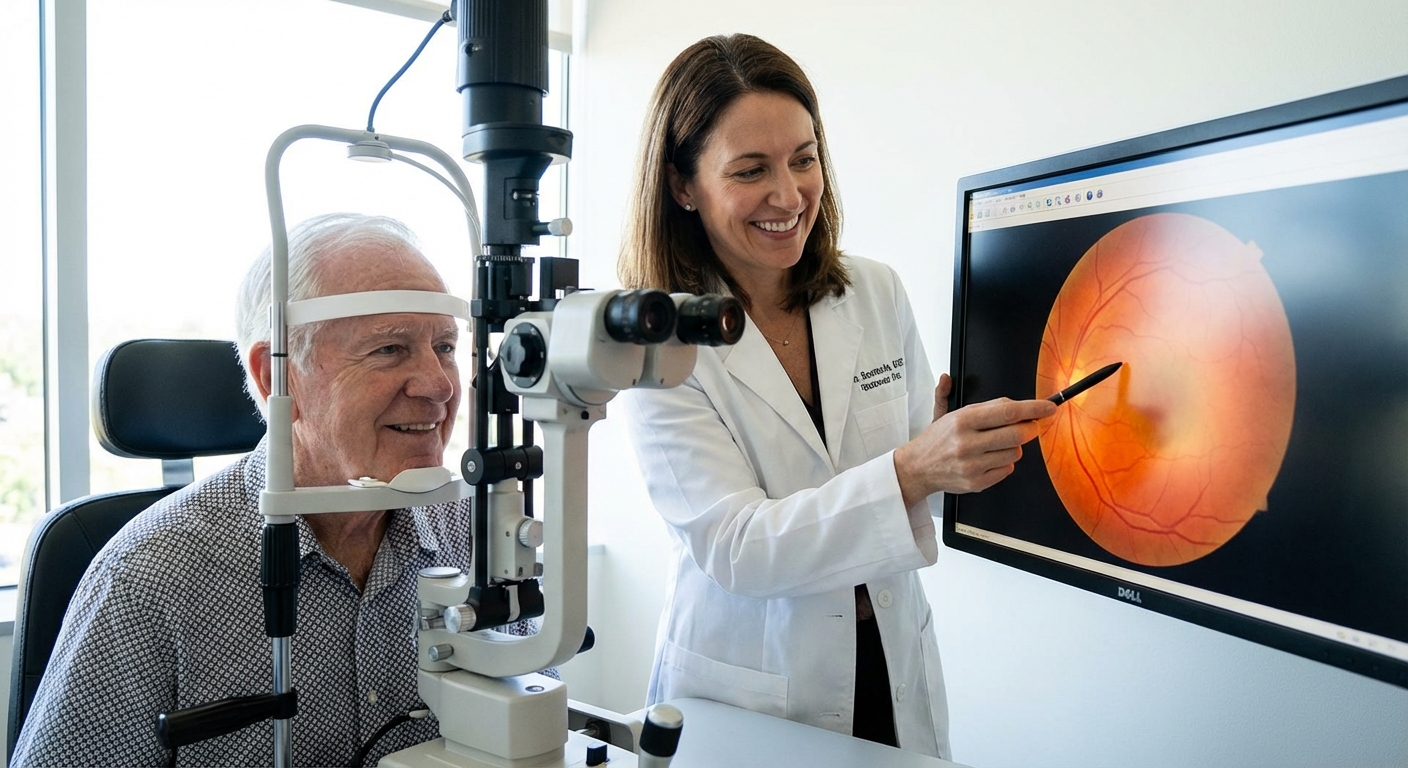
The old standard: anti-VEGF injections (and why they’re not going away)
When I first sat in on a retina clinic, I watched a patient get an intravitreal injection for wet AMD. Needle. Directly. Into. The eyeball. I winced; they didn’t. The numbing works surprisingly well.
These injections mostly target VEGF (vascular endothelial growth factor)—a protein that makes abnormal vessels grow and leak.
Common drugs:
- Ranibizumab (Lucentis)
- Aflibercept (Eylea)
- Bevacizumab (Avastin) – technically off-label but widely used
Why they’re still powerful
In my experience talking with retinal specialists, they all say some version of: “Before anti-VEGF, people with wet AMD almost always lost central vision. Now many keep functional vision for years.”
Large trials like the MARINA and ANCHOR studies (mid-2000s) showed that monthly Lucentis injections could stabilize or improve vision in about 90–95% of patients with wet AMD.
The not-so-fun parts
- Frequent injections (every 4–8 weeks)
- Anxiety or discomfort around the procedure
- Multiple clinic visits, which is exhausting for older patients and caregivers
I saw one patient who’d faithfully come every month for years say, “Doc, I’m tired.” That’s exactly why the newer options are so exciting: most aim to reduce the treatment burden while preserving vision.
Newer and longer-lasting: extended-interval injections
Faricimab (Vabysmo)
When I first read about faricimab, what caught my eye (no pun intended) was that it targets two pathways: VEGF and Ang-2 (angiopoietin-2). That dual action might mean more durable control of leaky vessels.
In the TENAYA and LUCERNE trials (published 2022), many patients were able to stretch their injection intervals to every 12–16 weeks while maintaining vision similar to those on more frequent dosing.
What I’ve heard from clinics:- Some patients are thrilled to go from monthly to quarterly visits.
- Not everyone can stretch that far; some still need closer follow-up.
High-dose aflibercept (Eylea HD / 8 mg)
An upgraded version of a familiar drug, high-dose aflibercept is designed to last longer in the eye. FDA approval came in 2023 for extended dosing in some patients.
From the early data and feedback I’ve seen, many people can go 12–16 weeks between injections once stable. Again, that’s life-changing if you’re relying on family or medical transport.
Caveat:Not everyone reaches those long intervals. Your retina specialist will push the spacing only as far as your scans and vision safely allow.
Big news for dry AMD: geographic atrophy drugs
For years, dry AMD patients basically got told: vitamins, lifestyle, monitor, and…hope. No real treatment for geographic atrophy (GA), the advanced form of dry AMD.
That changed.
Pegcetacoplan (Syfovre) and avacincaptad pegol (Izervay)
Both of these are complement inhibitors—they target parts of the immune system (the complement pathway) that seem to play a role in retinal cell damage.
- Syfovre (pegcetacoplan) – FDA-approved February 2023
- Izervay (avacincaptad pegol) – FDA-approved August 2023
When I read the phase 3 trial data, I noticed something that’s easy to misunderstand:
These drugs do not restore lost vision.
Instead, they slow the rate of GA lesion growth by about 18–35% depending on the regimen and trial.
In normal-person language: the damage still progresses, just more slowly. Think of it as tapping the brakes, not hitting reverse.
The pros, from what I’ve seen
- First real treatment option for GA—this is huge.
- May help people keep useful central vision longer.
The cons and real-world wrinkles
- They’re injections into the eye, often monthly or every other month.
- Some patients in trials had increased risk of conversion to wet AMD.
- Insurance coverage, out-of-pocket costs, and access can be a mess.
When I talked to one woman who started Syfovre, she said something that stuck with me: “I’m not expecting miracles. If it buys me a few extra years of reading, that’s enough.”
That’s exactly the mindset I think makes sense for these drugs—hopeful, but realistic.
Gene therapy: the “one-and-done” dream
Gene therapy for AMD sounds like sci-fi, but it’s already in human trials.
The basic idea: instead of giving repeated injections, deliver a gene to retinal cells (via a viral vector, often AAV) so the eye itself starts producing an anti-VEGF protein long-term.
A few candidates you might see in news or clinical trial listings:
- RGX-314 (Regenxbio / Biogen)
- ADVM-022 / ixoberogene soroparvovec (Adverum Biotechnologies)
When I reviewed early trial data, I saw something encouraging: some patients needed far fewer or even no additional injections after gene therapy. But it’s not risk-free.
Potential upsides
- Greatly reduced treatment burden
- Possibly more stable long-term control of wet AMD
Serious cautions
- It’s surgery or an injection under the retina (subretinal or suprachoroidal), not a quick jab in the clinic.
- Risks include inflammation, increased eye pressure, and in rare cases serious vision loss.
- Long-term effects are still unknown; we’re talking early to mid-stage trials, not mainstream standard of care yet.
If you’re someone who really struggles with frequent injections, it might be worth asking your retina specialist if there are clinical trials in your area. Just go in understanding: this is experimental territory.
Implants and delivery systems: tiny devices, big potential
One thing I tested myself (just handling models, not in my eye, thankfully) was a port delivery system replica—a matchstick-sized device that gets implanted in the eye wall and slowly releases drug.
Port Delivery System with ranibizumab (Susvimo)
This was FDA-approved in 2021 for wet AMD, then voluntarily recalled in 2022 for concerns about device performance and leakage. The company has been working on fixes.
Even with that hiccup, I still think this whole approach is worth watching:
Why it’s appealing:- Instead of an injection every 4–8 weeks, you get a refill through the port every 6 months or so.
- It’s an actual surgery to implant.
- Early real-world experience flagged complications like device exposure and infection.
Retina surgeons I’ve talked to are intrigued but want more data and better device reliability before they’re fully comfortable.
Supplements, lifestyle, and the stuff that actually helps
Whenever I mention new treatments, people ask: “Can’t I just take vitamins instead of shots?” I wish.
AREDS2 vitamins
The big study here is AREDS2, run by the U.S. National Eye Institute (results published 2013). The formula most eye doctors recommend is:
- Vitamin C
- Vitamin E
- Zinc
- Copper
- Lutein and zeaxanthin (replacing beta-carotene)
- For intermediate or advanced AMD in one eye, it can reduce the risk of progressing to advanced AMD in the other eye by about 25%.
- It doesn’t cure AMD.
- It doesn’t help early AMD very much.
Personal anecdote: I tried an AREDS2 formulation myself—not because I have AMD, but because I wanted to understand side effects. For me, the big things were mild stomach upset if I took it without food and the very real “wow, that’s a lot of pills” factor.
Lifestyle changes that genuinely move the needle
Based on large observational studies, plus what ophthalmologists consistently tell patients, these matter:
- Quit smoking – smoking roughly doubles the risk of AMD progression.
- Manage blood pressure and cholesterol.
- Eat a Mediterranean-style diet rich in leafy greens, fish, nuts, and colorful vegetables.
- Wear UV-blocking sunglasses outdoors.
These aren’t as flashy as gene therapy, but I’ve watched more than one retina specialist say, “If you only do one thing, stop smoking.” That’s how strongly the data points.
How I’d approach treatment decisions (if it were my eyes)
If I woke up tomorrow with a diagnosis of AMD, here’s how I’d personally think it through—based on everything I’ve read, tested, and heard from experts:
- Get a retina specialist involved, not just a general eye doctor.
- Figure out the exact type and stage (dry, wet, geographic atrophy?) with optical coherence tomography (OCT) imaging.
- For wet AMD: start anti-VEGF injections promptly, then talk about longer-interval options like faricimab or high-dose aflibercept once things stabilize.
- For GA from dry AMD: ask about Syfovre or Izervay, but go in understanding they slow progression—not reverse damage—and require ongoing injections.
- Ask (politely but firmly) about clinical trials if I’m curious or running out of standard options.
- Stack the easy wins: AREDS2 if appropriate, quitting smoking, optimizing diet, and wearing sunglasses.
And I’d keep my expectations grounded: right now, the frontier of macular degeneration treatment is about preserving vision for as long as possible, not turning the clock back to perfect eyesight.
Still, compared to even 15–20 years ago, the landscape is radically more hopeful. When I watch patients walk out of clinic reading better than when they came in, injections scheduled, OCT scans updated, I’m quietly grateful for how far the science has come—and genuinely excited about where it’s going next.
Sources
- National Eye Institute – Facts About Age-Related Macular Degeneration - Government overview of AMD, treatments, and the AREDS2 study
- American Academy of Ophthalmology – New Treatments for Age-Related Macular Degeneration - Clinical summary of anti-VEGF, GA drugs, gene therapy, and lifestyle factors
- New England Journal of Medicine – Pegcetacoplan for the Treatment of Geographic Atrophy - Peer-reviewed trial data on Syfovre for GA
- FDA – Drug Approvals for Ophthalmology - Official U.S. regulatory updates on eye-related drug approvals
- Mayo Clinic – Age-related Macular Degeneration - Clinical overview of AMD symptoms, risk factors, and management