Learn About Invasive Ductal Carcinoma Diagnosis and Treatment Options
ing through her online chart, both of us pretending we totally understood what we were looking at.
We didn’t.
What I’ve learned since then—through helping her navigate appointments, pestering oncologists with way too many questions, and digging into the research—is what I’m sharing here. If you or someone you love just saw those three words, this is for you.
What Exactly Is Invasive Ductal Carcinoma?
When I first heard it, “invasive ductal carcinoma” (IDC) sounded almost sci‑fi. But it’s actually the most common type of breast cancer.
Here’s the simple version I wish someone had given us:
- “Ductal”: It starts in the milk ducts of the breast.
- “Carcinoma”: It’s a cancer of epithelial cells (the cells lining ducts and glands).
- “Invasive”: It’s broken through the duct wall and started invading nearby breast tissue.
According to the American Cancer Society, IDC accounts for about 70–80% of all invasive breast cancers. So when people say “breast cancer” in a general sense, they’re very often talking about IDC.
In my experience helping friends through this, the word invasive is the one that makes everyone’s stomach drop. But invasive doesn’t automatically mean “everywhere” or “incurable.” It just means the cancer isn’t entirely contained within the ducts anymore—it’s moved into surrounding breast tissue and could spread further if untreated.
Early Clues: Symptoms I’ve Actually Seen (and Missed)
Here’s the frustrating part: some people with IDC have no symptoms at all. Their cancer shows up on a routine mammogram before they ever feel a lump.
But I’ve also seen these signs in real life:
- A painless lump or thickening in the breast (what my friend noticed in the shower)
- A subtle change in breast shape or size
- Dimpling or puckering of the skin (kind of like orange peel)
- Nipple changes – turning inward, crusting, or unusual discharge
- Redness or swelling that just doesn’t go away
When I tested my own breast awareness skills after all this, I realized I hadn’t really been doing self-exams seriously. Now I at least know what my normal feels like, so I’d notice new lumps or weird changes faster.
No symptom list is perfect. Some of these can be caused by benign conditions. But if something feels off for more than a couple of weeks, it’s absolutely worth bringing up with a doctor.
How Doctors Actually Diagnose IDC
When our family friend first went in, we thought her quick ultrasound would give a yes/no answer. It didn’t. What I’ve since learned: no one is diagnosed with IDC based on imaging alone.
Here’s the usual diagnostic path, step by step.
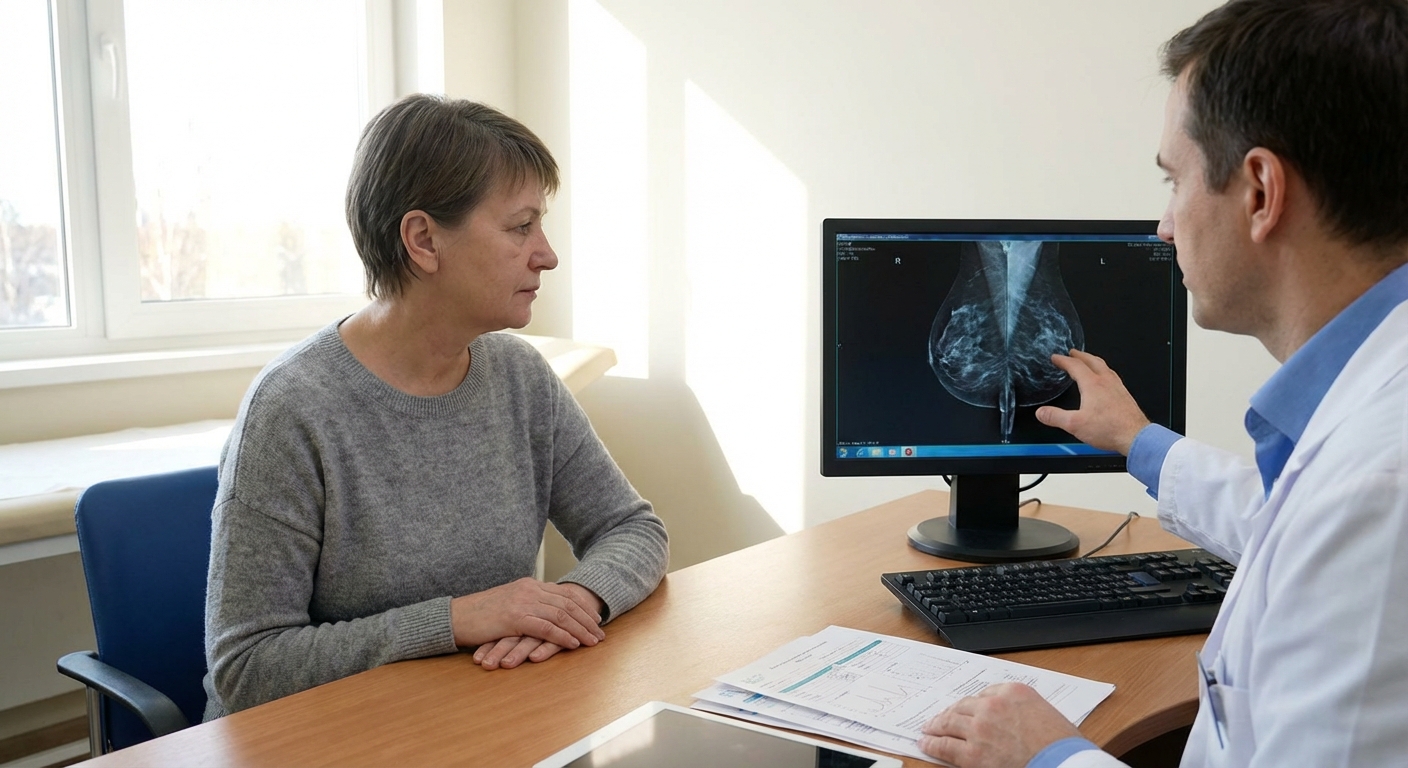
1. Imaging: Mammogram, Ultrasound, and Sometimes MRI
- Screening mammogram often spots a suspicious area or microcalcifications.
- Diagnostic mammogram zooms in for more detailed views.
- Breast ultrasound helps distinguish a solid mass from a fluid-filled cyst.
- Breast MRI is sometimes used for very dense breasts, high-risk patients, or to map the full extent of disease.
When I looked at a copy of the radiology report, I saw this mysterious “BI-RADS” number. That’s a standardized scoring system (0–6) used by radiologists. A BI-RADS 4 or 5 means there’s a suspicious abnormality and a biopsy is recommended.
2. Biopsy: The Definitive Answer
Every serious breast cancer conversation I’ve had with an oncologist started with the same sentence: “Diagnosis is made on biopsy.”
Common biopsy types:
- Core needle biopsy (most common): A hollow needle removes small cylinders of tissue.
- Vacuum-assisted biopsy: Uses suction to gather more tissue through a small incision.
- Surgical (excisional) biopsy: Removes all or part of the suspicious area.
The pathologist then looks at the cells under a microscope. If it’s IDC, the report will usually include:
- Histologic type: Invasive ductal carcinoma
- Grade (1–3): How abnormal and aggressive the cells look
- Hormone receptor status:
- ER (estrogen receptor)
- PR (progesterone receptor)
- HER2 status: A growth-promoting receptor that can be overexpressed
- Ki-67: A marker of how fast the cells are dividing
That one pathology report decides so much. When I first saw ER+, PR+, HER2– typed out, it looked like alphabet soup. Now I know: those 3 markers heavily shape treatment.
3. Staging: How Far Has It Spread?
Staging uses the TNM system:
- T (Tumor) – size and whether it’s grown into nearby structures
- N (Nodes) – has it reached lymph nodes?
- M (Metastasis) – has it spread to distant organs?
Your stage (0–IV) comes from combining these elements plus tumor biology. I’ve seen people with a small, low-grade Stage IA IDC have a very different conversation than someone with widespread Stage IV. Same diagnosis label, completely different reality.
Treatment Options: What Doctors Really Mean by “Personalized”
Here’s the big lesson I learned watching several people go through this: there is no one “IDC treatment plan.” There are patterns, yes, but oncologists mix and match based on stage, biology, and your health and preferences.
Surgery: Lumpectomy vs. Mastectomy
Most IDC treatment plans start with surgery, unless chemotherapy is given first.
- Lumpectomy (breast-conserving surgery): Removes the tumor plus a margin of normal tissue.
- Mastectomy: Removes the whole breast.
When I asked a breast surgeon bluntly, “Which is safer?” she pulled out the data: for many early-stage cancers, lumpectomy + radiation offers the same survival as mastectomy.
Pros & cons I’ve seen up close:
- Lumpectomy pros: Keeps most of the breast, often a shorter surgery, faster physical recovery.
- Lumpectomy cons: Usually requires radiation; sometimes more than one surgery to get clear margins.
- Mastectomy pros: Lower risk of local recurrence in the affected breast, may skip radiation in some cases.
- Mastectomy cons: Bigger surgery, longer recovery, major body-image impact, possible reconstruction decisions.
Most people also have a sentinel lymph node biopsy to see if cancer has spread to the first draining lymph nodes.
Radiation Therapy
Radiation is like the cleanup crew, targeting microscopic cancer cells left in the breast or chest wall.
You’re more likely to need it if you:
- Had a lumpectomy
- Had positive lymph nodes
- Had a larger tumor or close/positive margins
A typical schedule I’ve seen: daily treatments (Mon–Fri) for about 3–6 weeks. It’s painless during each session but can cause fatigue and skin irritation over time. One friend described it as “the world’s slowest sunburn.”
Hormone (Endocrine) Therapy
If your cancer is ER+ and/or PR+, hormone therapy is usually recommended.
Common drugs include:
- Tamoxifen (often for premenopausal women)
- Aromatase inhibitors like anastrozole, letrozole, or exemestane (often for postmenopausal women)
These drugs don’t kill cancer cells directly; they block estrogen’s ability to fuel their growth.
Upside: They significantly reduce the risk of recurrence for hormone-receptor-positive IDC.
Downside (from what I’ve heard repeatedly): hot flashes, joint pain, mood changes, vaginal dryness, bone thinning. The meds work, but they’re not always fun.
Chemotherapy
Chemotherapy is more likely to be recommended if the cancer is:
- Higher stage
- High grade
- Triple-negative (ER– / PR– / HER2–)
- Involves lymph nodes
Some people get neoadjuvant chemo (before surgery) to shrink the tumor; others get adjuvant chemo (after surgery) to reduce recurrence risk.
I’ve sat in infusion centers enough to know: chemo is intense. Hair loss, fatigue, nausea (much better controlled nowadays), increased infection risk—these are real. But for certain tumor types, it’s also a big survival booster.
Targeted Therapy for HER2-Positive IDC
If your tumor is HER2-positive, your oncologist will probably bring up drugs like trastuzumab (Herceptin) and pertuzumab (Perjeta).
Before the early 2000s, HER2+ breast cancer was considered more aggressive and harder to treat. Then trials like HERA showed trastuzumab dramatically improved outcomes. Now, HER2+ IDC is often very treatable with the right combo of chemo + targeted therapy.
These drugs specifically target the HER2 receptor on cancer cells, acting like smart missiles rather than carpet bombing.
Genomic Tests: Oncotype DX and Friends
One thing that really surprised me was how often genomic tests are used in early-stage, ER+, HER2– IDC to decide if chemo is necessary.
Oncotype DX, for example, looks at the activity of 21 genes and gives a recurrence score. Studies like TAILORx (NEJM, 2018) showed that many women with intermediate scores could safely skip chemo.When I saw a friend get a low score and legitimately avoid months of chemotherapy, it drove home how much more nuanced treatment has become.
Side Effects, Fear, and the Stuff People Don’t Put on Brochures
On paper, treatment plans look neat and logical. In real life, they’re messy.
Things I’ve seen people actually struggle with:
- Decision overload: “Lumpectomy or mastectomy?” isn’t just medical; it’s emotional and identity-level.
- Fatigue that lingers long after chemo or radiation.
- Lymphedema (arm swelling) after lymph node removal or radiation.
- Chemo brain – that weird mental fog that’s hard to quantify but very real.
- Anxiety around every follow-up scan.
And yet, I’ve also seen:
- People work through treatment part-time.
- Parents doing school pickups with chemo pumps under their sweaters.
- Survivors run 5Ks after radiation, not because they love running, but because finishing felt like flipping cancer the bird.
The reality is somewhere in the middle: neither “you’ll be fine, it’s routine” nor “your life is over” is true. It’s tough and doable and unfair and survivable—often all in the same week.
What I’d Do First If I Just Got an IDC Diagnosis
If I could time-travel back to that first stunned moment reading the pathology report with my friend, here’s what I’d push us to do:
- Get the full pathology report and ask the oncologist to explain: stage, grade, ER/PR/HER2, Ki-67.
- Write down every question before appointments. Your brain will go blank in the room; this helps.
- Ask directly about goals: “Is this treatment meant to cure, or to control the cancer long-term?”
- Get a second opinion—especially at a major cancer center—if something doesn’t sit right.
- Bring someone with you to appointments to take notes. Phone recordings (with permission) can also be lifesavers.
And maybe most importantly: remember IDC is common and heavily studied. That sounds cold, but it’s actually good news. Doctors have decades of data, better drugs than ever, and real evidence to guide decisions.
You don’t have to understand every acronym to advocate for yourself. You just have to keep asking, “Why this option for me?” until the answer makes sense.
Sources
- American Cancer Society – Invasive Breast Cancer (Invasive Ductal Carcinoma) - Overview of IDC, incidence, and characteristics
- National Cancer Institute – Breast Cancer Treatment (PDQ®)–Patient Version - Detailed, evidence-based summary of current treatment options
- Mayo Clinic – Invasive Ductal Carcinoma - Clinical explanation of IDC and common management
- New England Journal of Medicine – TAILORx Trial Results - Landmark study on using genomic testing (Oncotype DX) to guide chemotherapy
- CDC – What Is Breast Cancer? - General background on breast cancer types and risk factors